


Next: Analysis of Local
Up: Variable Gain Control in
Previous: Experiments
The key long-range model results depend on the properties of the local cortical
circuitry, so we begin by reviewing and expanding on these results.
We have recently described a local circuit model of orientation
selectivity in V1, which accounts for the results of most intracellular,
extracellular and pharmacological studies of orientation selectivity
[38]. This model requires only weakly oriented thalamic inputs
and utilizes
recurrent, short-range excitatory connections between neurons to amplify
and sharpen orientation selectivity. Inhibition contributes indirectly
to tuning by balancing excitation within the columnar
population. In this model cortical neurons retain
sharp orientation tuning independent of stimulus
contrast
[1,14,35,36].
This physiological property cannot be
achieved solely by the broadly oriented thalamocortical inputs used in the model
(see
[38]), but rather requires cortical inputs. Typical
contrast response curves generated by the model (figure 1)
closely resemble
experimental curves from V1 neurons
[1,4,11].
One key property of this cortical module is that
cortical excitation amplifies low contrast responses, while cortical inhibition
grows strong at high contrasts to attenuate responses (e.g.,
contrast saturation).
Thus this cortical circuitry not only provides sharp orientation
tuning but also yields a dynamic gain control mechanism: recurrent
excitation strongly amplifies preferred responses at low levels of
thalamic drive while inhibition reduces amplification or even attenuates
preferred responses at high levels of thalamic drive. Several differential
properties of excitatory and inhibitory neurons and synapses contribute
within a local feedback circuit to generate this gain
regulation as a network property.
Fast-spiking inhibitory interneurons exhibit higher firing rates and
greater frequency vs. current slopes, due to the higher input resistance
and brief after-hyperpolarization potentials of inhibitory neurons
[27].
Inhibitory neurons due to their lower surface areas appear to receive
fewer synaptic inputs and thus may have higher contrast thresholds.
Other possible differences between excitatory and inhibitory neurons may also
exist, but have not been considered here.
Such a variable gain control mechanism appears well suited to contribute
to other reported cortical response nonlinearities within the classical
receptive field. For example, cortical neuron responses decline over a
broad range of stimulus velocities for which LGN responses
increase
[30].

Figure 1: Cortical gain control of local circuitry.
Response amplitude
is shown as a function of stimulus contrast for an uncoupled cortical
cell (dashed red line) and for a cortical cell with short-range cortical
excitatory and inhibitory connections (solid blue line). Cortical connections
generate a contrast-response function with greater slope (high gain) at
low contrasts and stronger response saturation (low gain) at high contrasts
than the uncoupled cell.
Cortical gain control arising from local connections is also
the substrate for receptive field dynamics that arise from long-range
horizontal connections. We have constructed a 1:40 scale model of
the cortical circuitry under a 2.5 mm by 5 mm region of cortical surface. The
model contains over 20,000 spiking neurons with approximately 1 million synaptic
connections. (A preliminary version has been reported previously
[37]).
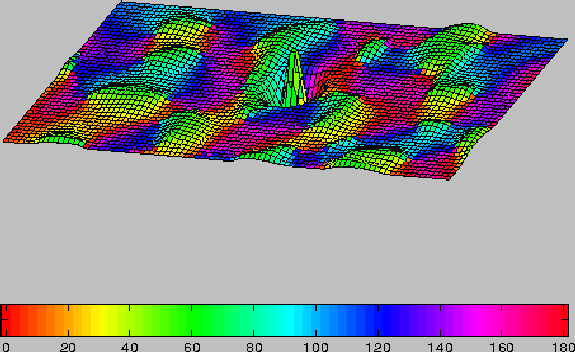
Figure 2:
Long-range and short-range connectivity of the model.
Columnar orientation preferences are shown on the
color map (see key). Mesh elevation represents the strength
of total synaptic connections ( Excit - Inhib ) to cells of the central
(green) orientation
column. Long-range synapses (excitatory only) generally connect
domains with similar orientation preference. Short-range connections
consist of both excitatory
and inhibitory inputs with excitatory inputs stronger at shortest distances
and inhibitory inputs stronger at greater distances.
Long-range connections originate from excitatory
neurons and target both excitatory and inhibitory neurons with similar
orientation preferences (see methods; figure 2).
Large scale computer simulations
were employed to compare neuronal responses to oriented grating stimuli that
covered (a) the classical receptive field alone (``center'' stimuli),
(b) the classical and extraclassical receptive fields
(``center + surround'' stimuli). Center contrast in both cases was
varied systematically from zero to high, while the iso-orientation
surround grating was kept at high contrast. The surround grating modulated
center responses in a way that depended on the contrast of the center grating.
Responses to low contrast center gratings were facilitated by the
presence of a high contrast surround grating, while responses to high
contrast center gratings were suppressed by the same high contrast
surround grating (figure 3).
The surround stimulus, and thus the long-range
inputs, were the same for both low and high contrast centers; however,
these inputs had opposite effects due to the gain control state of the
local cortical circuitry. Thus, the model predicts that fixed-strength
long-range connections can have bi-phasic modulatory effects, depending
on the strength of the central excitatory drive.
Although both kinds of modulatory
effect could be produced by surround gratings that differed in orientation
from the center grating, due to weaker long-range inputs the effects
were substantially weaker than for iso-orientation gratings
(simulation results not shown).
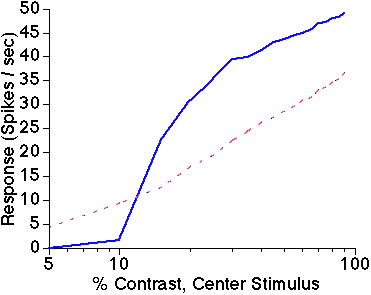
Figure 3: Biphasic surround modulation.
Average response amplitude of 144 excitatory neurons
in the central iso-orientation domain is shown as a function of center
stimulus contrast. Solid blue line is response to center stimulus alone;
dashed red line is response to center plus high contrast surround stimulus.
The presence of the iso-orientation surround stimulus facilitates responses
to low contrast center stimuli, but suppresses responses to high contrast
center stimuli.



Next: Analysis of Local
Up: Variable Gain Control in
Previous: Experiments





